Risk Factors for Incident Infections During Hepatitis C Treatment
Maciej S. Jabłkowski, Michael Melia, Erik Pulkstenis, Mani Subramanian, Mark Sulkowski
Med Sci Tech 2014; 55:31-36
DOI: 10.12659/MST.889286
Available online: 2014-04-25
Published: 2014-04-25
Background:
Incident infections are common during treatment of chronic hepatitis C virus (HCV). Risk factors for infections, however, have not been well-characterized. To define these risk factors, we analyzed infections that arose in the ACHIEVE 1 and ACHIEVE 2/3 studies comparing the efficacy and safety of ribavirin plus either albinterferon alfa-2b (albIFN) or pegylated interferon alfa-2a (PEGa-2a) for the treatment of chronic HCV infection.
Material and Methods:
2597 patients with genotype 1, 2, or 3 HCV infection were treated with ribavirin (RBV) plus either PEGa-2a 180 µg weekly (n=864), albIFN 900 µg every two weeks (n=873) or albIFN 1200 µg every two weeks (n=860). Data on infections were collected and graded by study investigators according to standard criteria. Risk factors for infections were analyzed by baseline and on-treatment demographic, laboratory, and disease characteristics. Independent predictors of infection were modeled using logistic regression analyses.
Results:
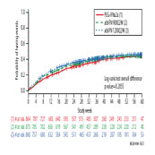
40–42% of patients in each group developed infections, most of which involved the respiratory tract. There were no significant differences in rates of infection across treatment groups. The majority of infections were mild-to-moderate; severe and serious infections arose in 3–4% of patients. Development of grade 3 or 4 neutropenia or lymphocytopenia was not associated with increased infection risk. On multivariate analysis, only baseline BMI <25 (OR=1.46, 95% CI 1.02–2.10, p=0.039) and on-treatment hemoglobin <10 g/dL (OR=1.81, 95% CI 1.25–2.61, p=0.002) were associated with incident infection.
Conclusions:
Infections are common among patients treated for chronic HCV with interferon and ribavirin, although serious and severe infections are rare. Low baseline BMI and on-treatment development of hemoglobin <10 g/dL are associated with an increased risk of infection, whereas incident neutropenia and lymphocytopenia do not increase infection risk. These findings have important implications for the management of side effects of HCV therapy.
Keywords: ribavirin, infections, Hepatitis C, albinterferon alfa-2b, pegylated interferon alfa-2a



